by Chris Gorini, PhD, Research Director & Jenna Jubeir, Intern
Background
Chronic spinal pain is a debilitating problem that affects more than 80% of the population and is the leading cause for physical and emotional suffering, disability, and missed work days.1,2 Treatment for relief of neck or back pain often relies on surgery, physical therapy, or a combination of both. These traditional treatments may prove ineffective or, in some cases, increase pain and prevent individuals from enjoying their lives. On treatment option for alleviation of chronic spinal pain is spinal cord stimulation (SCS). SCS is a technique that allows for continuous delivery of a low-voltage, electrical current to the spinal cord in order to block pain signals. If a patient is unresponsive to conventional treatments for chronic spinal pain, SCS may be a viable alternative.
History & Function
SCS first surfaced as a new approach for pain management in 1967 and was approved in 1989 by the Food and Drug Administration (FDA) to relieve chronic pain due to nerve damage (neuropathic pain). Technology has since advanced to more closely tailor patients’ needs. To understand the basics of SCS, it is helpful to first understand how your brain perceives “pain”. The spinal cord is comprised of bundles of nerves that are responsible for sending both sensory and motor signals to and from your brain, respectively. Sensory nerves run throughout your body and traverse the spinal cord before reaching the brain. Think of the spinal cord as transportation hub; the signals generated from your peripheral nerves in your wounded finger, for example, are then transferred (via the spinal cord) to another nerve that runs to your brain. Once this signal is received by your brain, it is then interpreted as a painful sensation. This all happens in a matter of milliseconds. In the case of individuals with chronic pain their sensory nerves are constantly sending electrical signals to the brain’s pain center. Similarly, when a nerve in your spine is damaged due to an accident, trauma, or disease, the damaged nerve elicits an electrical signal via sensory nerves, sending a “pain” signal to your brain. This pain can radiate across your limbs making it extremely difficult to live a normal, productive life.
Although pain is generally considered to be a protective response, chronic pain can present despite no visible indications that something is
wrong. SCS is typically used for neuropathic pain which normally does not serve a protective purpose and does not support healing.3 The actual mechanism by which SCS relieves pain is still under debate, although it is thought to function via a ‘gate’ theory. The gate theory explains how the central (brain and spine) and peripheral (nerves outside the brain and spine) nervous systems work in conjunction to communicate to the brain that one is experiencing pain. Once the pain signal is generated vi periphery (cut finger, damaged nerve, etc.), it encounters a type of ‘gate’ in the spinal cord. The gate is responsible for determining the extent to which the signal travels through the spinal cord up to the brain. By sending an outside electrical signal through the nerves responsible for the pain generation, one is able to ‘close’ the pain gate and minimize the pain signals that reach the brain.1,4 The main goal of SCS is to inhibit painful sensor signals at the spine and cut off the relay to the brain. Sixty years after the first use of a stimulator, the exact mechanism of SCS-derived pain relief is still discussed. However, it is now clinically proven that, with minimal side effects, SCS is effective in relieving chronic pain by changing how our bodies interpret and react to pain signals.
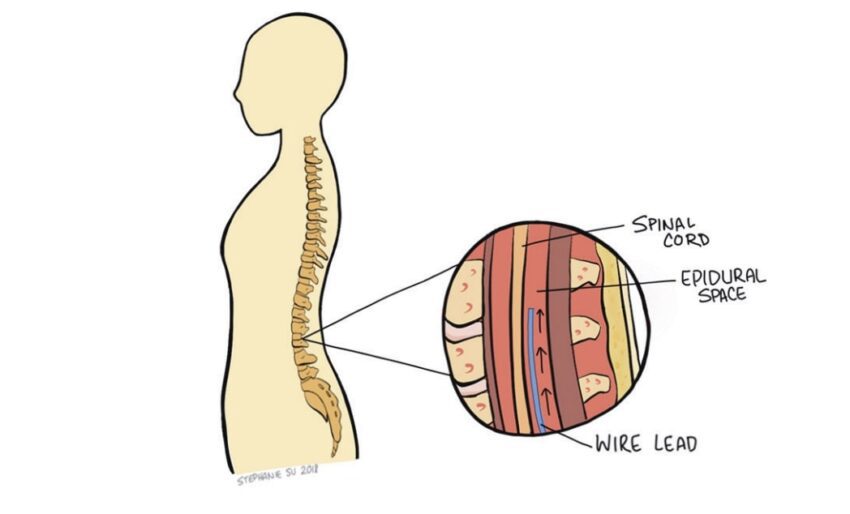
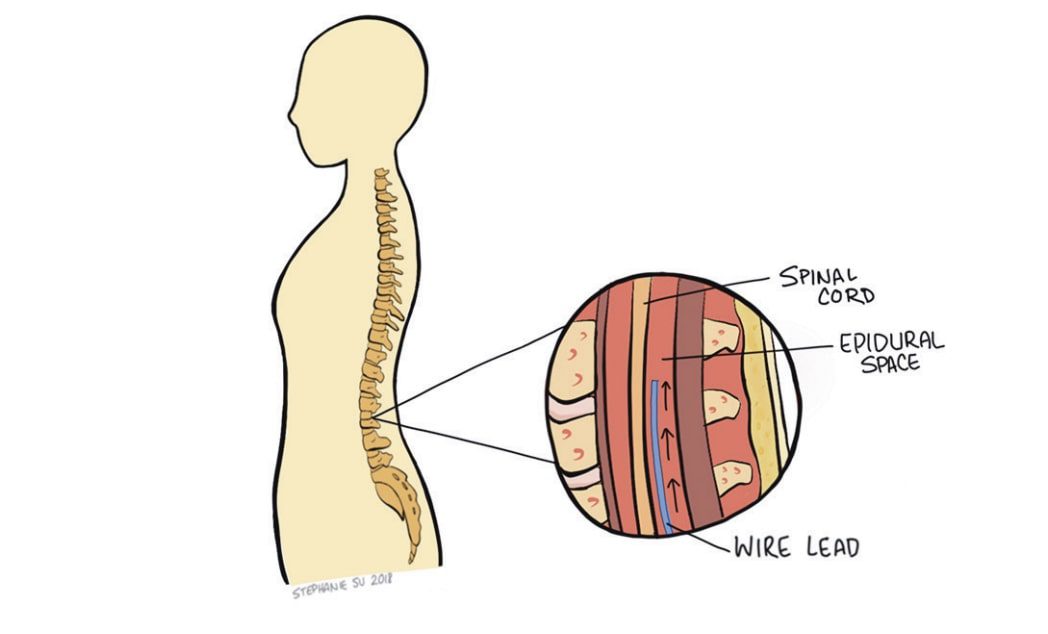
Traditional Spinal Cord Stimulation
The standard SCS technology used today has four main components: electrode leads, an extension wire, a pulse generator, and a wireless handheld remote. The pulse generator (roughly the size of a pocket lighter) generates the electrical impulses responsible for intercepting the pain signals. Upon generation, the pulses flow through the extension wire to the leads implanted around the nerves of the spine. The physician and patient may control the output of impulses via remote and may adjust a variety of parameters including amplitude, frequency, and pulse width. Spinal cord stimulators use one of two types of electrode leads: paddle or cylindrical. Each has their unique set of advantages and disadvantages. Both deliver impulses through uniformly spaced electrodes, allowing for a relatively large area to be covered. However, there are different implantation procedures depending on which lead is chosen. Cylindrical leads can be placed percutaneously (through the skin) and only require local anesthesia. While cylindrical leads are mobile within the body and are easier to adjust, they also move more freely and could stray from their intended target nerve, failing to effectively relieve pain. Paddle leads are implanted through more invasive surgeries requiring direct access to the spinal cord. Because paddle leads are anchored to the tissue, it is rare for the leads to move. However, fixing the leads within the epidural space makes them more difficult to manipulate after placement if any adjustments are needed. The location of the leads is one of the main determinants of pain relief. Despite variances between lead fixation methods, studies indicate little difference in painrelief between the two SCS systems.5 Use of spinal cord stimulators always starts with a trial period of 3-15 days. This is meant to test the efficacy of SCS on the patient before executing a more permanent implantation. Leads are first placed percutaneously with the pulse generator externally taped to the patient. While the patient is awake, the leads are adjusted to determine the ideal location for maximum pain relief. This s often accomplished using a series of real-time x-rays. The physician then adjusts the electrical pulse for optimal pain relief. At the end of the trial period, the physician decides whether to permanently implant the spinal cord stimulator based on the patient’s self-reported outcome. Most practices proceed with SCS if the patient experiences at least a 50% reduction in pain.6 If the patient decides to move forward, leads are placed (depending on type of leads chosen) around the nerves of the spine, in the area of interest, and the pulse generator is surgically implanted in the lower back/buttocks for security. With the hardware permanently in place, patients are able to participate in a variety of activities without worrying about dislodging equipment.7 The battery life of the pulse generators continue to improve, with some lasting over a decade.1 Moreover, with the adaptation of new technology, patients are able to securely adjust their pulse generation parameters using Bluetooth, often from their Smartphone. This allows the patient to increase or decrease the power (within range) of the electrical signal depending on their pain without having to return to the physician’s office. SCS has had promising success in relieving pain, increasing quality of life, and decreasing opioid usage for chronic pain patients. A 20-year survey reported that using SCS for Complex Regional Pain Syndrome had an 84% success rate of alleviating 50% or more of pain. The success rate of SCS for back/limb pain and failed back surgery syndrome showed a 67% and 62% success rate, respectively. Additionally, research conducted on opioid usage before and after SCS concluded that the morphine equivalent dose of pain medications used by patients decreased after the SCS systems were implanted, giving a promising look at an alternative to opioids.
Complications
The majority of complications associated with SCS can be prevented or reversed. The most common complications are hardware problems such as lead migration, where the leads move within the patient’s epidural space. One study found that 17% of patients experienced lead migration, however, with proper physician expertise, this is becoming less common. The next most widespread complication, lead breaking, occurs in only 7% of patients and requires a repeat procedure to repair.6 With the advancement of
materials and technology, lead malfunction will become less common. Spinal cord stimulation is not a risk-free procedure, but with the current research and success rates, over 50,000 people worldwide opt for SCS each year, with the vast majority experiencing life-changing relief.
The Future of Spinal Cord Stimulation
New SCS technologies are continually being approved by the FDA and utilized in practices across the United States. The top four medical device companies in the SCS industry (Abbott, Boston Scientific, Medtronic, and Nevro) have been competing for decades to create the most affordable, effective, and innovative devices. Industry is working on manufacturing SCS devices that aim to avoid paresthesia, which some find uncomfortable. Higher frequency and burst pulse generation are two options currently being explored. High-frequency (HF) devices use impulses with a frequency of 10,000 Hz instead of the traditional 50 Hz. A new study using these HF SCS devices show a 25% higher efficacy rate in reducing chronic back pain compared to traditional SCS systems. Burst SCS avoids paresthesia in a different way. These systems send five rapid pulses through the leads that mimic the body’s natural way of firing electrical signals, preventing the patient from feeling a numbness or tingling.
Conclusion
With new research and technology continuing to surface, SCS will only become more effective at relieving chronic pain. In the coming years, perfecting SCS technology and monitoring prescription drug usage will continue to minimize the prevalence of chronic neck or back pain and decrease dependence on opioids.
Interested in learning more? Be sure to visit our journals here!